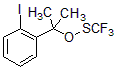
Trifluoromethylthio group is one of the most lipophilic groups. Introducing it into small molecule compounds can significantly enhance the ability of molecules to penetrate lipid membranes and increase the absorption rate in the body (Figure 1). Therefore, incorporating trifluoromethyl sulfide (CF3S) into drug candidates has become an important strategy for drug discovery; an effective way is to use a simple, stable, and efficient trifluoromethylthiolation reagent to directly introduce trifluoromethyl sulfide in the later stage of synthesis. Sulfur group.
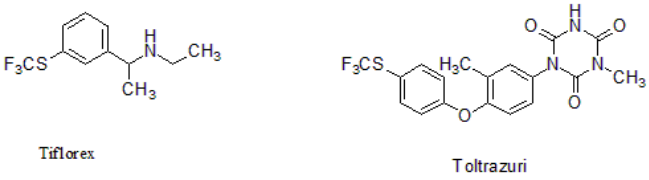
Trifluoromethyl sulfide (CF3S) is incorporated into drug candidates Tiflorex and Toltrazuri to enhance the molecule's ability to penetrate lipid membranes and increase the rate of absorption in the body
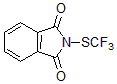
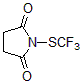
J&K Scientific sells two new types of trifluoromethylthiolation reagents developed by Shen Qilong’s team at the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences—N-trifluoromethylthiosaccharin (Shen Reagent) and trifluoromethyloxysulfide (Lu -Shen Reagent)
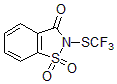
1. N-trifluoromethylthiosaccharin (Shen Reagent)
Solid product, low toxicity: Compared with the simplest and commonly used electrophilic methylthiolation reagent, trifluoromethylsulfoxide chloride (gas), it avoids the difficulty and danger of gas participating in the reaction, and greatly reduces the toxicity;
The substrate has a wide range of applications and is suitable for the trifluoromethylthiolation of alcohols, amines, thiols and electron-rich aromatics, aldehydes, ketones and β-ketoesters. It is suitable for the trifluoromethylthiolation of a variety of compounds. Provides possibilities (Figure 2);
The reaction speed is fast, the conditions are mild, and the yield is high (Table 1):
Functional groups are well tolerated.

Shen's trifluoromethylthiolation reagent functionalizes nucleophiles such as alcohols, amines, thiols, electron-rich aromatics, aldehydes, ketones, acyclic β-keto esters and alkynes to form trifluoromethyl sulfonating reagents. Fluoromethylthio (-SCF3) derivatives
Table 1: N-trifluoromethylthiosaccharin (Shen Reagent) has fast reaction speed, mild conditions and high yield.
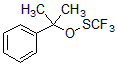
It complements N-trifluoromethylthiosaccharin (Shen Reagent) substrate and has the following characteristics:
Thermal stability, water stability;
Broad substrate range: Trifluoromethyl sulfide for aryl Grignard reagents, aryl and alkylboronic acids, alkylcarboxylic acids, alkynes, indoles, β-ketoesters, 2-substituted oxyindole derivatives and sodium sulfite base (Figure 3).

- Chunfa Xu, Bingqing Ma, and Qilong Shen; Angew. Chem. Int. Ed. 2014, 53, 9316 –9320
- Xinxin Shao, Chunfa Xu, Long Lu, Qilong Shen; J. Org. Chem. 2015, 80, 3012−3021
- Xinxin Shao, Xueqiang Wang, Tao Yang, Long Lu, Qilong Shen; Angew Chem Int Ed. 2013, 52, 3457-3460
- Xinxin Shao, Chunfa Xu, Long Lu, Qilong Shen; Acc Chem Res. 2015, 48, 1227-1236
Other trifluoromethylthiolation reagents