Heterocalixarene and crown arene are two new types of synthetic macrocyclic main molecules. Heterocalixarene has a finely adjustable 1,3-alternating conformation structure and V-shaped macrocyclic cavity [1], and crown arene has a cylindrical shape. Heterocalixarenes and crownarenes composed of cavities, different heteroatoms, aromatic rings and aromatic heterocycles exhibit rich and unique molecular recognition properties and the ability to form high-valence metal-organic compounds. Therefore, these two types of macrocyclic host molecules are widely used in the selective identification and detection of transition metal ions, organic cations, anions, and multiple types of central molecules, as well as carbon dioxide adsorption, stationary phase separation analysis, macrocyclic molecular liquid crystals, and organic-metallic molecules. Preparation of frameworks and metal cluster materials and study of the mechanism of metal-catalyzed aromatic carbon-hydrogen bond activation reactions.

General structural formula of heterocalixarene

The general structural formula of crown aromatic hydrocarbons
The research group of Professor Wang Meixiang of Tsinghua University has developed a series of new heterocalixarene and crownarene compounds after years of painstaking research. Bailingwei is honored to cooperate with this research group to commercialize heterocalixarene and crownarene compounds to facilitate supramolecular chemistry research. They have the following advantages:
Diverse structures: Provide new macrocyclic platform molecules. These compounds are the most basic skeletons and can be used for various derivatizations, making up for the poor structural modification of the second-generation supramolecular host compound cyclodextrin;
Unique performance: unique molecular recognition performance and sensing performance, filling the gap in the recognition and sensing of neutral molecules, transition metals, and anions by the first-generation supramolecular host compounds crown ether and cryptanether;
Wide range of applications: used in the construction of hard materials (MOFs), soft materials (liquid crystals) and functional materials, biometric identification, diagnosis, drug sustained release, etc., supplementing the shortcomings of traditional calixarene applications.
heterocalixarene
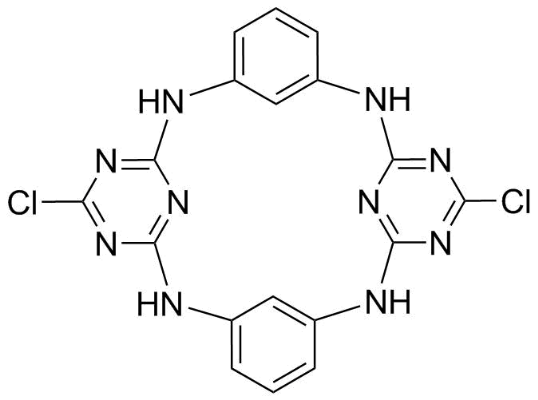
Application: Triazine macrocyclic molecular platform, molecular self-assembly primitives and liquid crystal materials for hydrogen bonding network [1,2,3,8]
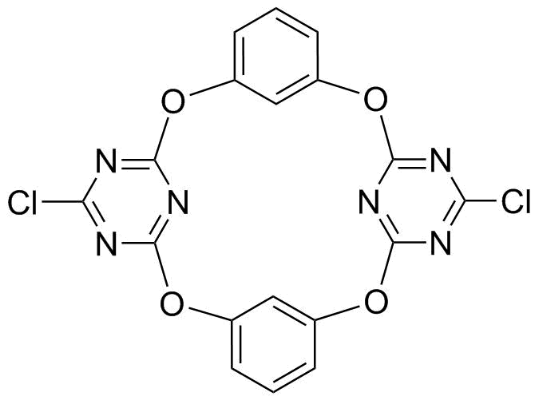
Application: Macrocyclic molecular platform, excellent macrocyclic host molecule for studying anion-π non-covalent interactions [1,2-7]

Application: Hydrogen bond acceptor, potential macrocyclic Lewis base, can be used as transition metal, heavy metal, anionic ligand [1,9-12]
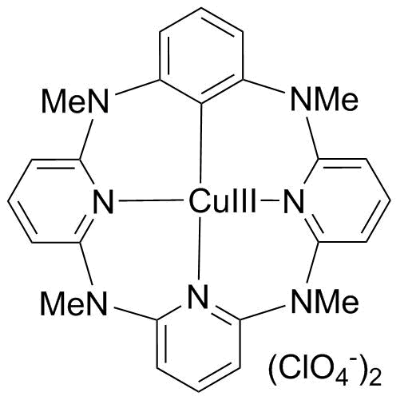
Application: High-priced copper chemistry: such as copper catalysis and copper-mediated organic reactions [1,13-16]
Crown aromatics
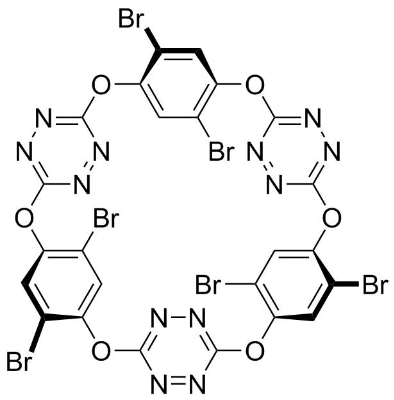
Application: Functionalized macrocyclic molecular precursors containing electron-deficient cavities for ion recognition, ion channel research, and coupling reactions [17,18]
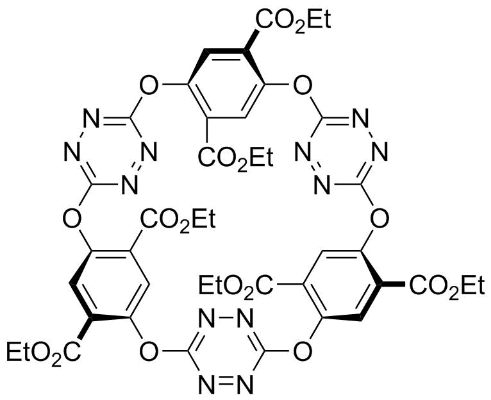
Application: Water-soluble macrocyclic molecular precursor containing electron-deficient cavities, used for ion recognition and ion channel research, coupling reactions [17,18]
参考文献
- Mei-Xiang Wang. Acc. Chem. Res. 2012, 45, 182-195.
- Mei-Xiang Wang, Hai-Bo Yang. J. Am. Chem. Soc. 2004, 126, 15412-15422.
- Hai-Bo Yang, De-Xian Wang, Qi-Qiang Wang, Mei-Xiang Wang. J. Org. Chem. 2007, 72, 3757-3763.
- Bao-Yong Hou, De-Xian Wang, Hai-Bo Yang, Qi-Yu Zheng, Mei-Xiang Wang. J. Org. Chem. 2007, 72, 5218-5226.
- De-Xian Wang, Qi-Yu Zheng, Qi-Qiang Wang, Mei-Xiang Wang.Angew. Chem. Int. Ed. 2008, 47, 7485-7488.
- Qi-Qiang Wang, De-Xian Wang, Hai-Bo Yang, Zhi-Tang Huang, Mei-Xiang Wang. Chem. Eur. J. 2010, 16, 7265-7275.
- De-Xian Wang, Mei-Xiang Wang.J. Am. Chem. Soc. 2013, 135, 892-897.
- Qi-Qiang Wang, De-Xian Wang, Hong-Wei Ma, Mei-Xiang Wang. Org. Lett. 2006, 8, 5967-5970.
- Mei-Xiang Wang, Xiao-Hang Zhang, Qi-Yu Zheng. Angew. Chem. Int. Ed. 2004, 43, 838-842.
- Han-Yuan Gong, Qi-Yu Zheng, Xiao-Hang Zhang, De-Xian Wang, Mei-Xiang Wang. Org. Lett. 2006, 8, 4895-4898.
- Han-Yuan Gong, Qi-Yu Zheng, Xiao-Hang Zhang, De-Xian Wang, Mei-Xiang Wang. Chem. Eur. J. 2006, 12, 9262-9275.
- Han-Yuan Gong, De-Xian Wang, Jun-Feng Xiang, Qi-Yu Zheng, Mei-Xiang Wang. Chem. Eur. J. 2007, 13, 7791-7802.
- Bo Yao, De-Xian Wang, Zhi-Tang Huang, Mei-Xiang Wang. Chem. Commun. 2009, 2899-2901.
- Hu Zhang, Bo Yao, Liang Zhao, De-Xian Wang, Bo-Qing Xu, Mei-Xiang Wang. J. Am. Chem. Soc. 2014, 136, 6326-6332.
- Bo Yao, Yang Liu, Liang Zhao, De-Xian Wang, Mei-Xiang Wang. J. Org. Chem. 2014, 79, 11139-11145.
- Chao Long, Liang Zhao, Jing-Song You, Mei-Xiang Wang. Organometallics, 2014, 33, 1061-1067.
- Qing-Hui Guo, Zhan-Da Fu, Liang Zhao, Mei-Xiang Wang. Angew Chem. Int. Ed. 2014, 53, 13548-13552.
- Mei-Xiang Wang. Sci. China Chem. 2018, 61, 993-1003.
