The disulfide skeleton containing two covalently linked sulfur atoms is an important molecular structure in life sciences, medical sciences, and food chemistry due to its unique pharmacological and physicochemical properties (Figure 1). For example, disulfide bonds in biomolecules play various roles in various biochemical redox processes; the secondary and tertiary structures of proteins also pass through disulfide bridges. In recent decades, people have discovered effective bioactive natural products and drugs with sulfur-sulfur bonds, such as the antifungal polycarpamine family and the anti-poliovirus epidhitodiketoprazine (ETPs) family. In terms of antibody-drug conjugates (ADCs), disulfide bonds are also widely used as linkers to introduce active drugs into target cells after cleavage after internalization of the ADC. Since the concentration of free thiols (glutathione) in cells is higher than that in blood, sulfur-sulfur bonds can be selectively broken in the cytoplasm of cancer cells, achieving specific release of cytotoxic molecules [1].

Figure 1: The importance of disulfide scaffolds in life sciences, natural products, pharmaceuticals, antibody drug conjugates and food chemistry
Chemists have been hoping for a new method to efficiently synthesize disulfide compounds. So far, there are few efficient and practical methods to construct asymmetric supersulfur. In most cases, double prefunctionalized thiol (phenol) nucleophilic substitution is used (Figure 2). This requires more synthesis steps and causes side reactions due to two active sulfur derivatives; this has major problems in terms of atom economy, reaction compatibility, and later environmental protection treatment.
Professor Jiang Xuefeng’s scientific research results have been transformed, and Bailingwei launched a new disulfide reagent:

Figure 2: Traditional synthesis method of disulfides: double prefunctionalized thiol (phenol) nucleophilic substitution
One-step synthesis, efficient construction: no need to introduce S into both reactants, the disulfide structure can be introduced in one step (Figure 3);
Facilitates post-modification: can realize post-modification of a variety of drugs and life unit molecules (sugar, amino acids, oligopeptides, vitamins, sulfa drugs) (Figure 4);
Atomic economy, green and environmental protection

Figure 3: New method for disulfide synthesis: introducing disulfide structure in one step

Figure 4: Can realize post-modification of multiple drugs and life unit analysis
In the process of drug design, the steric hindrance and chirality ortho to the disulfide bond in sulfides significantly affect the efficacy and selectivity of the drug. In order to solve the problems of difficulty in the synthesis of chiral disulfide bonds and limited diversity of ortho groups, etc. , Jiang Xuefeng’s team developed N-disulfide phthalimide reagent, which can quickly construct disulfides with three-dimensional structures. The reaction conditions are mild, the substrate range is wide, and the freedom of steric hindrance ortho to the disulfide bond can be achieved. Transformation[3].

Figure 5: Steric disulfides can be constructed
Due to the low dissociation energy of disulfide bonds, tetrasulfides have been cleverly developed as building blocks of disulfides in recent years. Jiang Xuefeng's research group designed a series of cyclic disulfide reagents to construct a variety of asymmetric tetrasulfides, and then used the tetrasulfides to construct disulfides. This method has the characteristics of wide substrate range, mild conditions, and high efficiency [4 ,5].

Figure 6: Construction of tetrasulfide compounds using cyclic disulfide reagents

Figure 7: Constructing disulfides from tetrasulfides
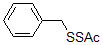
250MG 1G 5G

5G
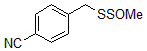
5G
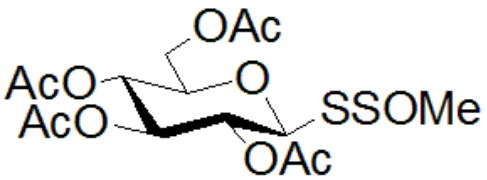
1G
References
- X. Xiao, J. Xue, X. Jiang, Nature Commun., 2018‚ 9‚ 2191.
- X. Xiao, M. Feng, X. Jiang, Angew. Chem. Int. Ed.,2016‚ 55‚ 14121-14125.
- Gao W C , Tian J , Shang Y Z , Chemical Science, 2020, 11.
- Xue J , Jiang X , Nature Communications, 2020, 11(1):4170.
- Xue J , Jiang X , Organic Letters, 2020, 22(20):8044-8048.
