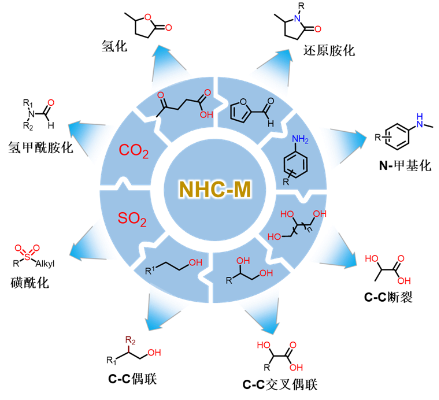
In recent years, transition metal catalysts coordinated by nitrogen heterocyclic carbene (NHC) have become a research hotspot for various cross-coupling reactions. Among them, Pd, Cu, Ag, Au and other metals are the main ones. They have high stability and can be used in various processes. Suzuki, Heck, Sonogashira and other cross-coupling reactions are performed under oxygen conditions.
Professor Tu Tao of Fudan University prepared a new type of nitrogen heterocyclic carbene metal catalyst, which enhanced the activity of the nitrogen heterocyclic carbene metal catalyst by increasing the conjugated aromatic ring of the NHC ligand and increasing the number of NHC ligands. This type of catalyst can be used in many catalytic reactions, such as oxidative dehydrogenation and coupling of alcohols, sulfonylation reactions with sulfur dioxide insertion, reductive amination reactions, etc. (Figure 1).
Figure 1 Numerous catalytic reactions involving nitrogen heterocyclic carbene metal catalysts
Bailingwei exclusively sells the scientific research results of Professor Tu Tao - a new nitrogen heterocyclic carbene metal catalyst. The product advantages are as follows:
Novel structure, new three types of (acenaphthyl, bis and triazacarbene) double-coordinated nitrogen heterocyclic carbene metal (Ir, Pd, Au) catalysts;
Wide range of applications, good catalytic activity and selectivity, can be used in some challenging reactions, such as oxidative dehydrogenation cross-coupling of alcohols;
The product is solid and easy to use and store.
Tu Tao, professor and doctoral supervisor in the Department of Chemistry, Fudan University. In recent years, his research interests have mainly focused on: 1. Preparing a series of stimulus-responsive metal-organic molecular gels through self-assembly strategies and applying them to supramolecular catalysis, recognition and cell imaging; 2. Based on metal nitrogen heterocycles Carbene compounds have the characteristics of high activity and good stability, with rigidity Imidazolium salts are used as precursors to prepare a series of stable, efficient, and reusable solid molecular catalytic materials through coordination assembly and hyper-cross-linking strategies. While achieving the advantages of catalysis and efficient loading, they also achieve the minimization of carbon dioxide, biomass and other resources. Applications in molecular high value and energy conversion. He has published more than 70 SCI papers as the corresponding author in internationally renowned academic journals such as Adv. Mater.; Angew. Chem.; ACS Catal.; Green. Chem.; Chem. Commun.; Org. Lett., and he has been cited more than a thousand times. , has been highly praised and affirmed by domestic and foreign peers.
Application examples
The triazoheterocyclic carbene iridium catalyst can efficiently and highly selectively catalyze the cross-coupling of ethylene glycol and methanol to produce lactic acid. The reaction can complete quantitative conversion within one hour, and the TOF is as high as 3660h-1. In addition, studies have shown that the reactivity increases with the number of nitrogen heterocyclic carbene ligands (Figure 2).

Figure 2 Nitrogen-heterocyclic carbene iridium catalyst: efficient catalysis of selective cross-coupling of ethylene glycol and methanol to prepare lactic acid
Product list
Palladium catalyst
gold catalyst
Iridium catalyst