Metal-catalyzed cross-couplings are widely used for the C-C bonds formation in organic synthesis, particularly for unsaturated scaffolds production. However, there were several challenges to develop a general method for the direct deoxygenative cross-coupling of free alcohols, using as native sp3-hybridized functional groups.
Challenges:
- the in situ cleavage of strong C–O bonds
- commercial availability of structurally-diverse alcohols
Recently, J&K Scientific is proud to provide the Deoxazole, which is a kind of N-heterocyclic carbene salts for carbon-carbon bond formation with aryl halide coupling partners on metallaphotoredox-based which free alcohols are activated in situ by.
Direct deoxygenative arylation of alcohols by Deoxazole

A general strategy for the cross-coupling of alcohols is enabled by the merger of NHC-mediated alcohol activation, photoredox catalysis, and nickel catalysis.
- capable of accommodating a wide range of primary, secondary, and tertiary alcohols, as well as pharmaceutically-relevant aryl and heteroaryl bromides and chlorides.

The success of alcohol deoxygenative cross-coupling relies on the facile NHC-mediated C–O bond homolytic cleavage.1
- A mild, robust and selective method
- Highly efficient in reactions, easy operation without separation and purification of the intermediates.
- Stable powders, easily storage and handling
Used for late stage deoxygenative arylation of drugs
Example: late-stage functionalization of Taxol

NHC, nitrogen-heterocyclic carbene; Ac, acetyl; Bz, benzoyl; Me, methyl; Ph, phenyl; t-Bu, tert-butyl; TBS, tert butyldimethylsilyl.
Reference:
Products:
Cat. No.:9222646, Deoxazole, 98% [CAS:1207294-92-5]

Cat. No.:9222647, Deoxazole-OTf, 98%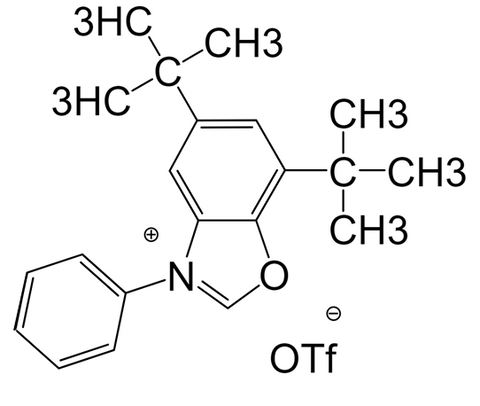
Cat. No.:9222648, Deoxazole-Quat, 98%

